Colon AiQ Test – Early Colon Cancer Detection
Colon AiQ offers the opportunity for those who need to be screened, but postpone colonoscopy or do not have access to it, to have a first thorough screening using molecular biology techniques to determine if there is a serious reason for an immediate colonoscopy.
It also provides an intermediate screening option for individuals with confirmed colon syndromes or a family history of cancer, where frequent screening is required, as they are in high-risk groups for developing colon cancer.


Colon AiQ Test – Early Colon Cancer Detection
Colon AiQ offers the opportunity for those who need to be screened, but postpone colonoscopy or do not have access to it, to have a first thorough screening using molecular biology techniques to determine if there is a serious reason for an immediate colonoscopy.
It also provides an intermediate screening option for individuals with confirmed colon syndromes or a family history of cancer, where frequent screening is required, as they are in high-risk groups for developing colon cancer.
A screening option for individuals postponing colonoscopy
According to Global Guidelines, all individuals over the age of 45 should undergo preventive screening for colon cancer.
However, there is a significant percentage of people who delay the preventive procedure of colonoscopy, usually due to its invasive nature. Colon AiQ test is the ideal solution to ensure that we do not neglect our preventive colon cancer checkup, helping us stay informed about our risk status.
Colon AiQ is the most accurate, non-invasive test in Europe for colon cancer, based on molecular biology and AI techniques.
Why should I choose the Colon AiQ test?
Colon AiQ
The Colon AiQ is an innovative, non-invasive test that utilizes genetic analysis of blood samples to detect specific DNA changes associated with the presence of colon cancer. Thanks to advanced technology for analyzing epigenetic biomarkers in liquid biopsy, Colon AiQ achieves higher sensitivity (accuracy of the positive result 86%) compared to the fecal immunochemical self-tests, especially in early stages of cancer, even at a stage where precancerous lesions (adenomatous polyps) are present.
This supports early colon cancer detection, allowing doctors to implement more effective treatment plans.
Self test (FIT - fecal immunochemical test)
Self test (FIT – fecal immunochemical test) is one of the most common methods for pre-symptomatic screening through the detection of microscopic traces of blood in the stool. However, since it only detects the presence of blood, it may not identify cancerous or precancerous lesions that do not cause bleeding, and the presence of blood could potentially be due to another cause (hemorrhoids).
Additionally, the higher sensitivity of the method seems to be limited to the more advanced stages of the disease, raising concerns about its ability to detect cancer in early stages.

How does Colon AiQ test work?
Colon AiQ is a non-invasive test that is performed on blood (liquid biopsy) and analyzes the methylation profile of 5 clinically validated genetic regions in free circulating genetic material – cfDNA (in the blood).
In the case of colon cancer, the methylation profile of these regions changes. By using this method, we can detect these changes at a very early stage of cancer (even at the stage of advanced adenoma, which is a precancerous lesion) and lead to an early diagnosis through colonoscopy.
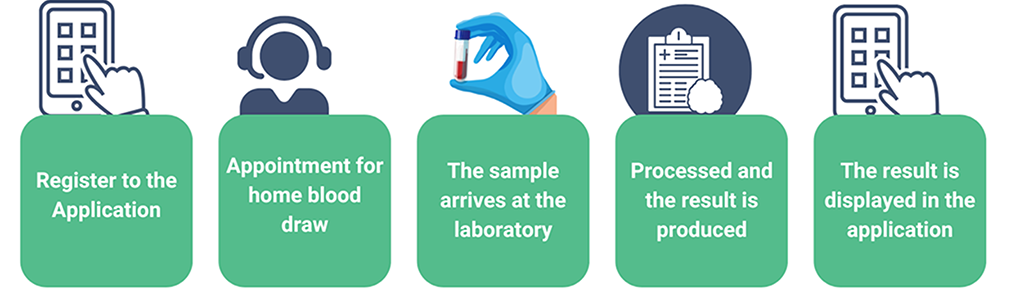
Procedure
The Colon AiQ test has 2 possible results






